Abstract
Introduction: Venetoclax (VEN), a selective BCL2 inhibitor, has demonstrated single-agent activity in patients with relapsed and refractory acute myeloid leukemia (AML) (Konopleva et al., 2016). In treatment-naïve elderly AML patients, encouraging safety and efficacy has also been established with the combination of VEN and the hypomethylating agents (HMA) azacitidine and decitabine (NCT02203773), as well as low-dose cytarabine (LDAC) (NCT02287233). VEN combination treatment strategies in relapsed/refractory AML and related hematologic malignancies have not been previously reported.
Methods: To determine the efficacy of VEN combinations in the salvage setting, we retrospectively reviewed all adult patients treated at our institution with myeloid malignancies (including AML, myelodysplastic syndrome (MDS), and blastic plasmacytoid dendritic cell neoplasm (BPDCN)) who received off-label VEN combination therapy outside of a clinical trial for relapsed/refractory disease. All patients receiving ≥ 14 days of VEN in salvage treatment combinations, from the time of VEN FDA approval for CLL (4/11/2016) to 5/8/17 were included. Responses were assessed by modified IWG criteria.
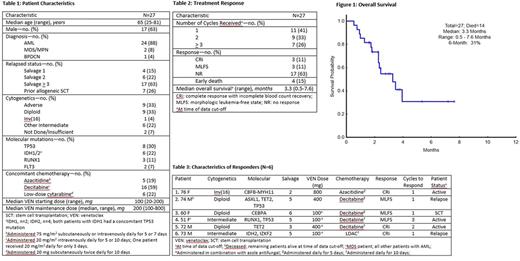
Results: Twenty-seven patients with relapsed or refractory AML, MDS, or BPDCN who received salvage treatment VEN combinations were identified (Table 1). The median age was 65 (range, 25-81). Most patients were treated for AML (88%, n=24), the remaining for MDS (8%, n=2) and BPDCN (4%, n=1). This relapsed population included 4 patients (15%) treated in the salvage 1 setting, 6 patients (22%) in salvage 2 and the remaining 63% (n=17) were salvage 3 or greater (range, 1-7). All but 5 patients had received prior HMA therapy; 7 patients (26%) had received a prior allogeneic stem cell transplant (SCT). At baseline, 33% (n=9) had adverse cytogenetics; 4% (n=1) had inv(16), 30% (n=8) had TP53 mutations, 22% (n=6) had IDH mutations (IDH1 : n=2; IDH2 : n=4), and 7% (n=2) had FLT3 mutations.
In combination with VEN, the majority of patients received either decitabine (59%, n=16) or azacitidine (19%, n=5). Six HMA-failure patients (22%) were treated with LDAC. Median VEN treatment dose was 200mg daily (range, 100mg-800mg); with dose reductions related to concomitant CYP3A4 inhibitor treatment, primarily azole antifungals, in 85% (n=22) patients. Tumor lysis syndrome prophylaxis was administered during the VEN "ramp-up" until the final dose was reached, during the first cycle.
Patients received a median of 2 cycles of therapy, (range, 1-6). Seven patients (26%) received 3 or more cycles. Objective response was achieved in 22% (n=6) patients after a median of 1 cycle (range, 1-4), including 11% (n=3) complete response with incomplete blood count recovery (CRi), and 11% (n=3) achieving morphologic leukemia-free state (MLFS) (Table 2). The 6 patients who achieved CRi or MLFS had all received ≥ 2 prior lines of therapy and 1 patient successfully proceeded to allogeneic SCT (Table 3). The patient with BPDCN had PET/CT major response and >50% bone marrow blast reduction, without obtaining formal response. Median survival at data cut-off was 3.3 months (range, 0.5-7.6) (Figure 1). Estimated 6-month survival was 31%. Four patients (15%) remain on VEN combination treatment. Of the 23 patients that discontinued therapy, reasons included progressive disease (n=16), AEs not related to progression (n=1), death (n=5) within the first 60 days, or transition to allogeneic SCT (n=1). Neutropenia and transfusion-dependent cytopenias were present in nearly all patients prior to the start of therapy, and continued on VEN-combination treatment.
Of 6 patients with IDH mutations, 1 patient experienced CRi. Two additional patients experienced >50% bone marrow blast reduction without full hematologic recovery, and an additional IDH-mutant patient rapidly cleared all peripheral blasts within the first 2 weeks of treatment but transitioned to hospice for infectious complications without a bone marrow response assessment. Two IDH mutant patients, including 1 with a concomitant FLT3-ITD mutation, had no evidence of response.
Conclusion: Low-intensity chemotherapy such as HMAs or LDAC in combination with VEN is a viable salvage treatment option, even in multiply relapsed/refractory patients with AML, MDS, and BPDCN. Responses were particularly notable in patients with diploid or otherwise intermediate cytogenetics and/or IDH-mutations.
DiNardo: AbbVie: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Agios: Honoraria, Research Funding. Takahashi: Symbio Pharmaceuticals: Consultancy. Jain: BMS: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Research Funding; Genentech: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Thompson: Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Pemmaraju: abbvie: Research Funding; affymetrix: Research Funding; Incyte Corporation: Consultancy, Honoraria; novartis: Consultancy, Honoraria, Research Funding; stemline: Consultancy, Honoraria, Research Funding; roche diagnostics: Consultancy, Honoraria; cellectis: Research Funding; LFB: Consultancy, Honoraria. Daver: Otsuka America Pharmaceutical, Inc.: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy; Pfizer Inc.: Consultancy, Research Funding; Jazz: Consultancy; Karyopharm: Consultancy, Research Funding; Bristol-Myers Squibb Company: Consultancy, Research Funding; Incyte Corporation: Honoraria, Research Funding; Sunesis Pharmaceuticals, Inc.: Consultancy, Research Funding; Daiichi-Sankyo: Research Funding; Immunogen: Research Funding; Kiromic: Research Funding. Jabbour: Bristol-Myers Squibb: Consultancy. Cortes: Sun Pharma: Research Funding; Pfizer: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; Teva: Research Funding; ImmunoGen: Consultancy, Research Funding. Kantarjian: Delta-Fly Pharma: Research Funding; Pfizer: Research Funding; Bristol-Meyers Squibb: Research Funding; Novartis: Research Funding; Amgen: Research Funding; ARIAD: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal